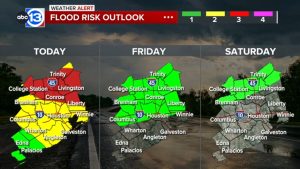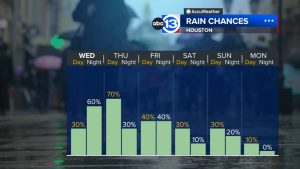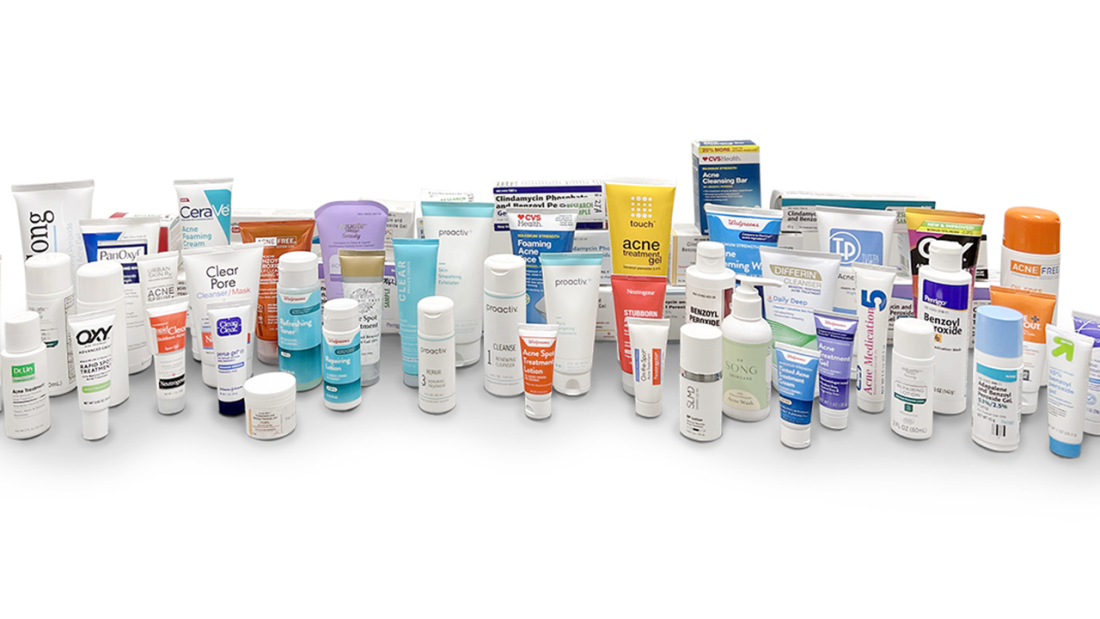
In a significant development, an independent laboratory petitioned the U.S. Food and Drug Administration (FDA) on Tuesday to recall a range of acne products suspected to contain heightened levels of benzene, a known carcinogen.
The lab, Valisure, based in Connecticut, raised alarm over the presence of benzene in these acne treatments, attributing it to the breakdown of benzoyl peroxide, a common ingredient found in over-the-counter topical acne remedies.
This isn’t the first time Valisure has flagged hazardous substances in everyday products. Over the past years, the lab has uncovered cancer-causing elements in various consumer goods and medications, prompting recalls, including benzene in hand sanitizers and spray sunscreens.
Benzene, classified as carcinogenic by numerous federal agencies and international organizations, poses significant health risks. Exposure to even low levels of benzene, as low as 0.8 parts per million (ppm), has been linked to various cancers, including blood and lymphatic cancers.
The FDA guidelines strictly prohibit the use of benzene in drug product manufacturing, advising against levels exceeding 2 ppm. Valisure’s testing revealed benzene contamination in 94 benzoyl peroxide products, often surpassing the permissible limit.
Moreover, Valisure’s research highlighted a concerning trend: a considerable spike in benzene levels when samples were subjected to common pharmaceutical stability testing temperatures, such as 122°F. This temperature mimics conditions encountered in everyday settings, indicating a potential hazard for consumers.
Further investigation by Valisure suggested that benzene could be leaching from product packaging, exacerbating the contamination risk. The lab posited that benzoyl peroxide products may degrade into gaseous benzene, dispersing into surrounding environments, such as hot cars or steamy bathrooms.
Beyond advocating for recalls, Valisure urged the FDA to scrutinize manufacturing processes of benzoyl peroxide products and develop robust testing protocols to detect benzene contamination. Brands implicated in Valisure’s findings include Proactiv, Target’s Up & Up, Clinique, Clearasil, and CeraVe.
The revelation underscores the urgent need for rigorous oversight and regulatory measures to safeguard consumer health. As the FDA evaluates Valisure’s petition, consumers are advised to exercise caution and remain vigilant when using acne products containing benzoyl peroxide, pending further regulatory action.